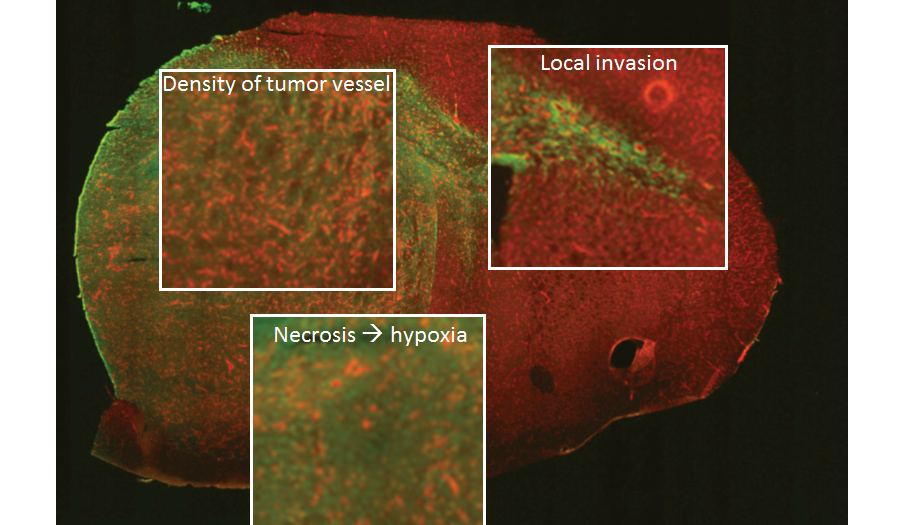
Projects related to low and high grade gliomas
(Group : Regulatory Networks of angiogenesis, Tumor Invasion and Metastasis)
Gliomas are brain tumours originating from glial cells and neural stem cells that surround and support neurons . They are classified on the basis of their clinical and histopathological characteristics to four grades with progressively more severe features. Among patients diagnosed with low-grade glioma (LGG) approximately 70% progress to grade IV glioblastoma multiforme (GBM) within 5-10 years of diagnosis . Primary glioblastomas are de novo GBM that are usually negative for the IDH mutation. Glioblastomas have both angiogenic and infiltrative growth pattern, that poses a challange to clinical mangement of the disease. In order to address the challenging questions raised above, we have established three different state-of-the art in vivo human GBM model systems, based using xenotransplantation in rodents, where adaptive processes can be studied in a sequential time-dependent manner. Using this approach, we have evidenced a mechanism to lead to an angiogenesis/invasion shift. This mechanism involves the unfolded protein response (UPR) triggers the shift from an angiogenic to invasive phenotype when one of the main UPR sensors is inhibited (Auf et al, 2010). This indicate that the UPR pathway constitutes one of the possible mechanistic pathways involved in the angiogenesis to invasion shift .
1/ Role of RhoGTPases in glioblastoma development :
We have more recently initiated large-scale approaches in animal models (RNAseq, proteomics etc..) where we are able to identify modules that distinguish low grade from high grade gliomas (Clarke, Daubon et al, Plos Genetics, 2015) (Figure 1). We use a data-driven approach to learn the structure of gene regulatory networks from observational experimental data and use the resulting models to formulate hypothesis on the molecular determinants of glioma stage. Remarkably, integration of available knowledge with functional genomics datasets representing clinical and pre-clinical studies reveals important properties within the regulatory circuits controlling low and high-grade glioma. Our analyses first show that low and high-grade gliomas are characterised by a switch in activity of two subsets of Rho GTPases. We are now focussing on specific molecular drivers that are important in the regulation of tumor cell infiltration using functional studies.
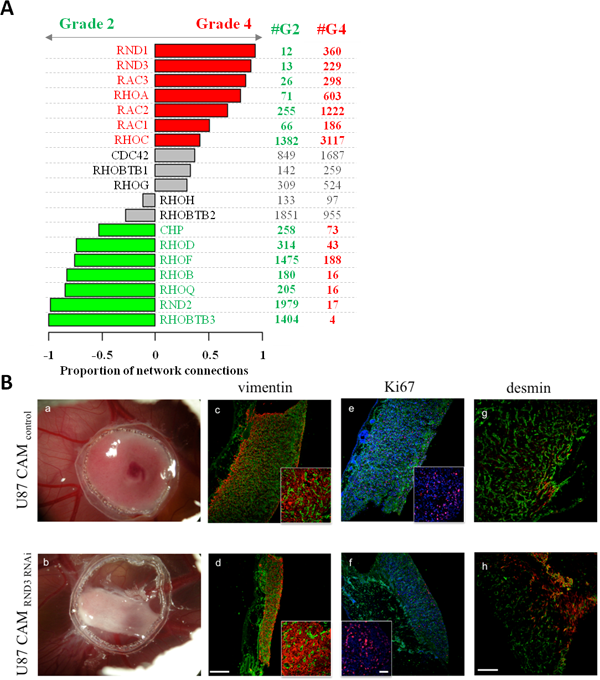 |
| Figure 1 : A : Analysis of RhoGTPases in grade II and IV gliomas B : Functional analysis of RND3 using knock-down in the experimental chick glioma model (Clarke, Daubon et al., Plos Genetics 2015). |
2/ Extracellular matrix in glioblastoma development :
ECM elements are central actors in the establishment of GBM cell invasion. I performed, after orthotopic implantation of cells derived from patients, xenograft sections (PDX) to extract tumor cells from angiogenic and invasive zones, by micro-laser capture. The RNAs were analyzed by total sequencing (messenger and non-coding RNA). Bioinformatic analyzes were performed to extract genes overexpressed in the invasive zones, coding for matrix proteins. One of these genes codes for thrombospondin-1 (THBS1) which was the transcript with the highest connectivity. THBS1 has been described as a strong anti-angiogenic molecule, especially in GBMs, but since then, some roles have been shown in apoptosis, cell cycle or invasion. Our hypothesis was that THBS1 promotes the acquisition of invasive features of GBM, however, its involvement in brain tumor cell invasion that has not been characterized in vivo so far. THBS1 has also been shown to activate TGFβ (Schultz-Cherry et al., 1994). TGFβ, as previously described, is a cytokine that causes major changes in cellular behavior, due to transcriptional modifications of target genes. Surprisingly, I was able to demonstrate that THBS1 was not a TGFb activator in GBM cells. On the other hand, stimulation with TGFβ1 upregulates the expression of THBS1 via Smad3 binding on its promoter. By using shRNAs against THBS1 in PDX models (Keunen et al., 2011), we observed a reduction in tumor size, or contralateral invasion in immunodeficient mice. In addition, a peptide approach allowing the inhibition of the binding of THBS1 to the CD47 receptor led, after treatment of mice implanted with the P3 cells, a decrease in the invasion of the cancer cells and in the tumor growth, respectively. This study, based on total RNA sequencing and then functional analysis using in vitro and in vivo experiments, allowed defining THBS1 as an essential element for tumor development and infiltration of cancer cells into surrounding brain tissues : this work is now published in Daubon et al, Nature Communications 2019.
Figure 2: 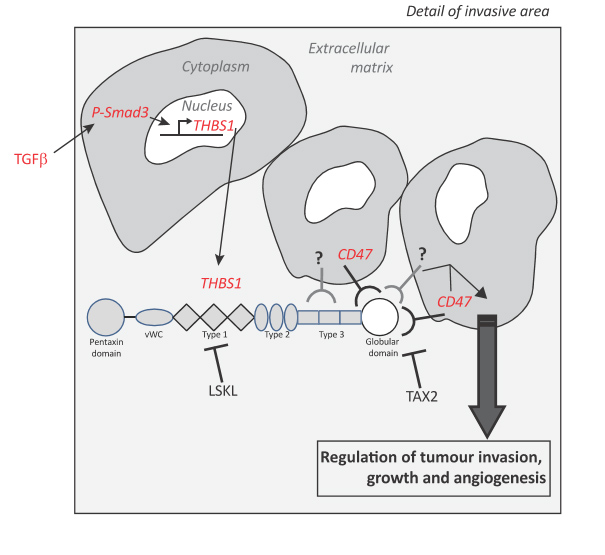
3/ Glioblastoma metabolism and microenvironment : putting tumor back in context
Glioblastomas are brain tumors that are derived from astrocytes or oligodendrocytes. These tumors have a heterogeneous structure composed of a necrotic and vascularized center and an invasive periphery. The rapid development of glioblastoma and its ability to invade surrounding tissues makes it a complex pathology to treat, and the average survival of patients ranging from 12 to 15 months. The first and second lines of treatment, based on the Stupp protocol, consisting of the resection of the tumor mass and adjuvant temozolomide treatment and/or radiotherapy, only allow a few months to delay recurrence. The additional use of anti-angiogenic treatment, despite its initial effects on tumor vascularization and the central tumor core, invariably leads to tumor escape. Tumor cell invasion is the central element in tumor recurrence. It is therefore important to understand the mechanisms of tumor invasion and understand the interactions between tumor cells and their microenvironment (astrocytes, blood vessels, neurons).I performed total RNA sequencing of the angiogenic and infiltrative areas of glioblastoma derived from patient xenografts. Data analysis in bioinformatics allowed us to identify molecules that may represent markers of tumor invasion. The acquired data sets also contained transcripts related to tumor cell metabolism, such as lactate deshydrogenase (LDH). These proteins are also found to be potentially essential molecules for regulating the neurovascular unit. My project has the following specific aims : 1/ Understanding the role of energy metabolism (LDHA, MCT1 etc.) in the interaction of tumor cells with the “normal” brain environment2 / Evaluation of the effect of therapy on cell metabolism and on the recurrence of glioblastomas. Identification of the molecules responsible for these effects.
A systematic analysis will be performed not only on LDHA, but also on LDHB. I will use knock-out and knock-down strategies as well as gain-of-function or pharmacological approaches. I will then analyze their impact on tumor development and invasion as well as on the tumor microenvironment, especially on the neurovascular unit. This study will lead to a better understanding of the role of LDHs in tumor cell invasion and the brain tumor microenvironment.
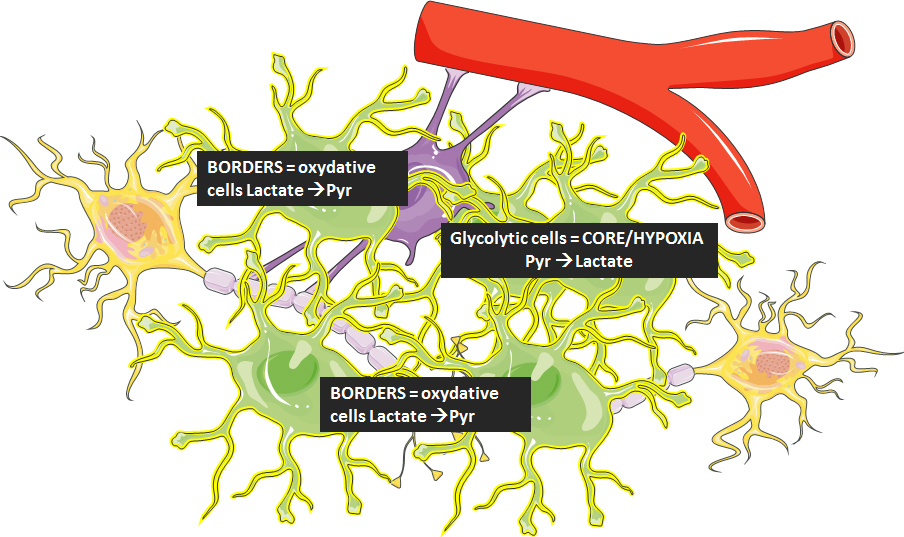 Figure 3
Figure 3
Project members :
– Dr. Thomas Daubon – Senior Post-doc and Project co-investigator
– Prof. Andreas Bikfalvi – Project co-investigator
– Joris Guyon, PhD student : Robo/Slit in glioblastoma invasion
– Dr. Gro Rosland : visiting scientist then Post-doctoral scientist from August 2019
– Tiffanie Chouleur : phosphatases and GBM + help on metabolism project
– Capucine (Rarahu) Magaut, PhD : phosphatases and GBM + help on membrane tube project
Visiting collaborators :
– Matthias Preussler : PhD student from Dresden University- Germany, visiting scientist in metabolism project
– Matteo Gambaretti : Medical student from Humanitas Hospital Milan-Italy, visiting scientist in low grade glioma ERANT project
Collaborations :
– Pr. Rolf Bjerkvig, University of Bergen : participant in ERANET grant
– Pr. Hrvoje Miletic, University of Bergen : co-financial support from Norwegian Cancer Society
– Pr. Lorenzo Bello, Department of Neurosurgery, Humanitas, Univ Milan.
– Dr Barbara Klink, Genetic Institute, Dresden University-Germany and LIH-Luxembourg
Current Research Fundings:
2019-2021 : Grant from Norwegian Cancer Society
2019-2020 : Abbvie financial support for ICV injection therapy project
2018-2019 : Financial support from ARTC for irradiation project
2017-2021 : ERANET grant on low grade glioma (ARC)
2018-2019 : Financial support from LLCC for GBM metabolism
2017-2018 : Financial support from LLCC for GBM heterogeneity
